U.S. Clot Management Market to Reach $3.4B by 2031 as Stroke & Embolization Tools Advance – iData Research
U.S. Clot Management Market to Hit $3.4B by 2032, Fueled by Neurovascular Innovation, Imaging Advancements, and AI-Guided Interventions
VANCOUVER, BRITISH COLUMBIA, CANADA, September 24, 2025 /EINPresswire.com/ -- A new 2025 U.S. Clot Management Device Market Report by iData Research reveals the market is projected to grow from nearly $2.8 billion in 2024 to over $3.4 billion by 2031, driven by neurovascular innovation, advanced embolization tools, and improved imaging for stroke and aneurysm intervention.
Visit iData’s 2025 U.S. Clot Management Report to explore how evolving treatment solutions are reshaping care across stroke, aneurysm, trauma, and oncology.
Produced by iData Research, a leading medical device market intelligence firm, this report offers strategic insights for neurovascular and peripheral vascular device manufacturers, marketing managers, product development teams, and procurement leads. It outlines how improved imaging, minimally invasive access tools, and novel biomaterials are expanding treatment options and reshaping purchasing criteria.
“The market is shifting toward tools that deliver more precise clot removal, safer navigation, and longer-term durability,” said Dr. Kamran Zamanian, CEO of iData Research.
Precision Embolization & Stroke Technologies Set New Standard in $3.4B Market
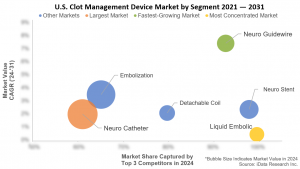
As neurovascular procedures become more specialized, manufacturers are introducing refined tools for safer navigation and improved outcomes. Detachable coils and intrasaccular stents are evolving with better conformability and flow-diverting properties, offering enhanced vessel remodeling and aneurysm sealing.
AI-powered stroke detection platforms and real-time imaging systems are accelerating treatment timelines and improving placement accuracy during interventions. In parallel, access tools like guidewires and catheters now offer better flexibility, trackability, and hydrophilic coatings, enabling safer passage through complex vasculature and reducing complications.
Meanwhile, the transcatheter embolization segment is expanding rapidly in both trauma and oncology, driven by advancements in embolic materials and delivery systems that allow for targeted occlusion with greater control and permanence.
The U.S. Clot Management Device Market, valued at $2.8 billion in 2024, is projected to exceed $3.4 billion by 2031, fueled by next-gen biomaterials, AI-guided interventions, and growing demand for minimally invasive stroke and embolization therapies.
Key Insights from the Report:
- AI and Imaging Integration Reshape Stroke Workflow: Platforms like Viz.ai and RapidAI are transforming stroke response with automated image analysis and faster triage, reducing time-to-treatment and improving outcomes in high-risk cases.
- Next-Gen Embolic Agents and Intrasaccular Stents Gain Ground: New flow-diversion and bioactive materials are redefining clot durability and vessel healing. Terumo’s FRED™ X and WEB™ systems highlight this shift, with U.S. adoption rising post-approval.
- Access Devices See Micro-Innovation: Microcatheters and guidewires are becoming smaller and more steerable, allowing safer navigation in delicate neurovascular anatomy. This supports broader use in aneurysm and AVM care.
- Peripheral Expansion Beyond Stroke: Transcatheter embolization is now routinely applied in trauma bleeding control and interventional oncology, expanding the addressable patient base and creating new market momentum.
- Endovascular Preference Over Surgery: Improvements in catheter durability and control are pushing clinicians toward less invasive approaches, reducing surgical burden while improving clinical and economic outcomes.
Who Should Read This Report?
This report is designed for marketing managers, CEOs, CFOs, as well as strategic decision-makers neurovascular and vascular intervention segments. It offers data-driven insights to support investment planning, R&D prioritization, and procurement optimization in the evolving clot management space.
Explore the Market Report
With stroke response tools and embolization technologies advancing rapidly, iData’s U.S. Clot Management Device Market Report provides the clarity needed to stay ahead.
Explore the full 2025 Report or request a sample to access revenue forecasts, pricing trends, competitive analysis, and clinical innovation updates.
Discover iData’s flexible Subscription Model at https://idataresearch.com/subscription-model/
With iData’s Subscription Model, teams can access multi-specialty device market data, including clot management, neurovascular, and embolization insights, all under a single, budget-aligned plan.
About iData Research
iData Research has been a leader in market intelligence for the medical device industry for over 20 years, delivering data-driven insights that help companies mitigate market risks, optimize pricing strategies, and uncover new revenue opportunities.
Learn more at https://idataresearch.com/
Maksym Brylkov
iData Research
+1 604-266-6933
email us here
Visit us on social media:
LinkedIn
YouTube
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.